Children ages 5-11 are now eligible to receive the Pfizer vaccine
DAVID BRUCE/ERIE TIMES-NEWS
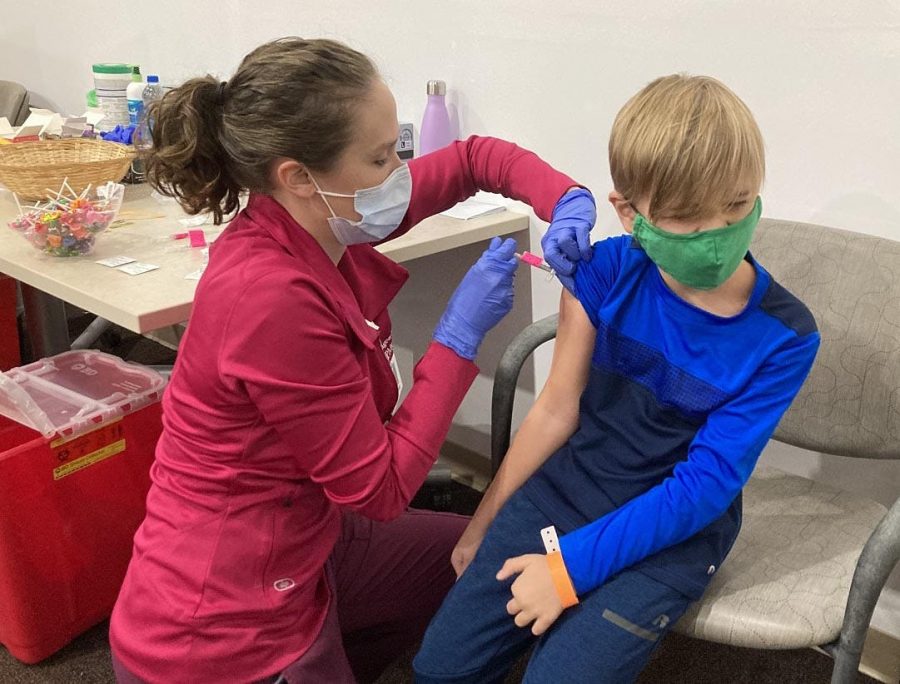
Kristin Lazzarra, R.N., gives a shot of Pfizer’s COVID-19 vaccine to Parker Schreffler, 7, of Lake City, at UPMC Health Plan Operations Center in Erie on Nov. 4.
November 16, 2021
On Tuesday, Nov. 2 with a vote of 14-0, the Center for Disease Control approved the Pfizer-BioNTech COVID-19 vaccine for children from ages 5-11. The availability of the vaccine for this age group opens up the chance for 28 million children to get vaccinated against COVID-19.
Since the vaccine is being labeled as a pediatric vaccine, all vaccine providers must use the pediatric vaccine formula: two 10-microgram doses, given 21 days apart. A major difference from vaccine administration for children ages 5-11 compared to adults is the one-third dose children get.
Children ages 5-11 can receive their vaccine at their local vaccine locations which include CVS, Walgreens and even elementary and middle schools. Broward County Public Schools is administering the vaccine at their schools to give more access for students to get the vaccine. This will aid kids who cannot receive the vaccine outside of school and possibly encourage more students to receive it.
The CDC recommends that everyone 5 years old and older receive the vaccination. Clinical trials in kids ages 5-11 presented the vaccine to be 90.7% effective in preventing symptomatic COVID-19 cases. The vaccine works to protect everyone who receives it against severe symptoms and death from the virus as well as the Delta variant.
“I feel that it is a good choice for children to receive the vaccine as long as everything has been properly tested, as it seems it has,” Marjory Stoneman Douglas High School sophomore Camilla Amador said.
A main concern parents have about vaccinating their child is possible vaccine side effects. While there have been a few cases documenting adverse reactions to the vaccine, scientific studies have shown the benefits outweigh the risk. In a trial of 3,000 vaccinated individuals, the data summarizes that all individuals experienced mild side effects like pain at the injection site, mild fatigue and headaches.
“This opening of the vaccine to younger citizens will help many since being in close contact with so many students increases the risk of getting COVID-19, so by vaccinating them, it can lower the chances of COVID-19,” freshman Jiya Anand said.
With children ages 5-11 gaining eligibility to receive the COVID-19 vaccination, the spread of the virus slows down since it provides more protection for children and the community. With the vaccination eligibility scope broadening, the hope is that COVID-19 cases, hospitalizations and deaths will continue to see a decrease.